Background: Individuals who have sickle cell disease (SCD) are susceptible to several severe consequences, such as vasoocclusive crisis (VOC), which may further develop into acute chest syndrome (ACS), a specific type of acute lung injury. Although VOC can result in substantial morbidity, it is important to note that ACS is the primary cause of mortality among individuals with SCD. Despite the devastating consequences of ACS, no interventions exist that directly treat this complication. Consequently, when patients present with ACS, their treatment options are frequently limited to supportive care. The absence of ideal therapeutic interventions for individuals with SCD who are experiencing ACS is a significant factor in their increased rates of illness and death. A cohort of adult patients diagnosed with ACS was examined with the objective to evaluate indicators of disease severity and identify risk factors associated with recurrent ACS.
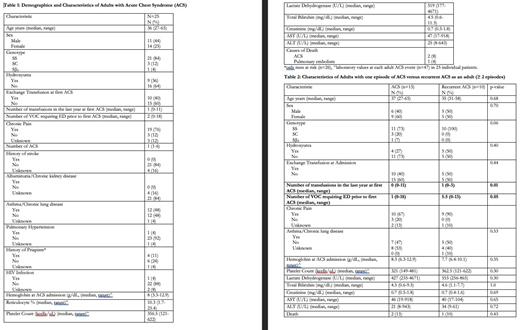
Methods: The Emory University Georgia Comprehensive Sickle Cell Center, located at Grady Health System, offers a dedicated acute care unit (ACU) that operates around the clock. This unit is specifically designed to assess and manage SCD patients who present with sickle cell crises. A pilot retrospective study was undertaken to analyze 25 patients with ACS. The evaluation included the examination of admission laboratory results, demographic information, past medical history, co-morbidity, interventions for each ACS admission for each patient (refer to Table 1). Descriptive statistics were used to report frequencies or median and range (Table 2). Patient characteristics of patients with 1 ACS event versus those with recurrent ACS (>=2) were compared using fishers exact test or the Wilcoxon-rank-sum test. Statistical significance was set at 0.05. Analysis was completed using SAS 9.4 (Cary, NC).
Results: A total of 47 ACS episodes were detected in a cohort of 25 individuals, resulting in a median of 1 ACS event per patient (range of 1 to 6). A total of 85% of the patients included in this study had homozygosity for SCD, whereas 36% of the patients were receiving hydroxyurea treatment. Chronic pain was reported in three-fourth of the cohort. Out of all the ACS events observed, only 40% necessitated red cell exchange transfusion. A correlation between a minimum hemoglobin (Hb) level of 3.3 g/dL and lactate dehydrogenase (LDH) levels over 4000 U/L was noted during ACS admission. These findings indicate the presence of intravascular hemolysis. Three individuals died to ACS. Next, we conducted a comparison between a group of 10 patients who experienced recurring ACS events, with a minimum of 2 to a maximum of 6 occurrences, and another group of 15 patients who only had a single ACS episode. The group of patients with recurrent ACS exhibits a median of 5.5 episodes of VOC that necessitate visits to the acute care unit within the year prior. In contrast, the group with a single ACS episode has a median of 1 event. There was a notable disparity in the frequency of transfusions received by patients with recurrent ACS compared to those who experienced only a single episode over the past year. Although no other variable demonstrated statistical significance, the recurrent ACS group exhibited lower levels of hemoglobin and higher levels of LDH in comparison to the group with a single ACS episode. Further examination of our patients with SCD could provide valuable insights into the potential factors that may predict ACS episodes. These factors include a patient's genetic background, the frequency of VOC, the use of hydroxyurea, evidence of hemolysis, and previous blood transfusions. Furthermore, the utilization of acute care unit by patients with SCD has had a positive effect in our cohort, on the reduction of red cell exchange transfusions and mortality rates from ACS, possibly from early identification and episodic transfusion, in comparison to similar cohorts with ACS.
Disclosures
Schoettler:Omeros: Consultancy, Honoraria; Alexion: Consultancy. McLemore:Novartis, Pfizer, NHLBI: Research Funding. El Rassi:Novartis, Forma Therapeutics, Agios: Research Funding. Chonat:Alexion: Consultancy, Other, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GBT/Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal